
Background: For patients (pts) with Philadelphia chromosome (Ph)-negative B-cell acute lymphoblastic leukemia (ALL), combination chemotherapy achieves complete remission rates of 80-90%; however, many pts ultimately relapse, leading to a cure rate of only 40-50%. Blinatumomab is highly effective in both the relapsed/refractory setting and for eradication of measurable residual disease (MRD). We hypothesized that early incorporation of blinatumomab in pts with newly diagnosed Ph-negative B-cell ALL would decrease the need for intensive chemotherapy, lead to deeper and more durable responses, and improve survival.
Methods: Pts 14-59 years of age with newly diagnosed Ph-negative pre-B-cell ALL, including pts who had received no more than 1 prior cycle of chemotherapy, were eligible. Pts were required to have a performance status of ≤3, total bilirubin ≤2 mg/dl and creatinine ≤2 mg/dl. Pts received hyper-CVAD alternating with high-dose methotrexate and cytarabine for up to 4 cycles, followed by 4 cycles of blinatumomab at standard doses. Pts with CD20+ disease (≥1% cells) received 8 doses of ofatumumab (2000 mg) or rituximab (375 mg/m2). Eight administrations of prophylactic IT chemotherapy were given in the first 4 cycles. Maintenance was with alternating blocks of POMP (given in maintenance cycles 1-3, 5-7, 9-11, and 13-15) and blinatumomab (given in maintenance cycles 4, 8, and 12). After 2 pts with high-risk features experienced early relapse prior to blinatumomab administration, for pts #10+ the protocol was amended so that pts with high-risk disease features (e.g. CRLF2+ by flow cytometry, complex karyotype, KMT2A rearranged, low-hypodiploidy/near triploidy, TP53 mutation, or persistent MRD) started blinatumomab after 2 cycles of hyper-CVAD.
Results: 39 pts have been treated, 34 of whom are evaluable for efficacy (5 too early for assessment). 6 pts were in complete remission (CR) at enrollment and unevaluable for morphologic response. Pt characteristics of the 34 evaluable pts are summarized in Table 1. Median age was 36 years (range, 17-59 years). At least one high-risk feature was present in 19 pts (56%), including TP53 mutation in 27%, CRLF2+ in 20%, and an adverse-risk karyotype in 26%. 82% of pts received ofatumumab or rituximab.
Among 28 pts with active disease at study entry, 100% achieved CR, with 82% achieving CR after the first cycle. MRD negativity by 6-color flow cytometry was achieved in 20/23 responding pts (87%) after 1 cycle and 33/34 pts (97%) overall. There were no early deaths, and the 60-day mortality rate was 0%.
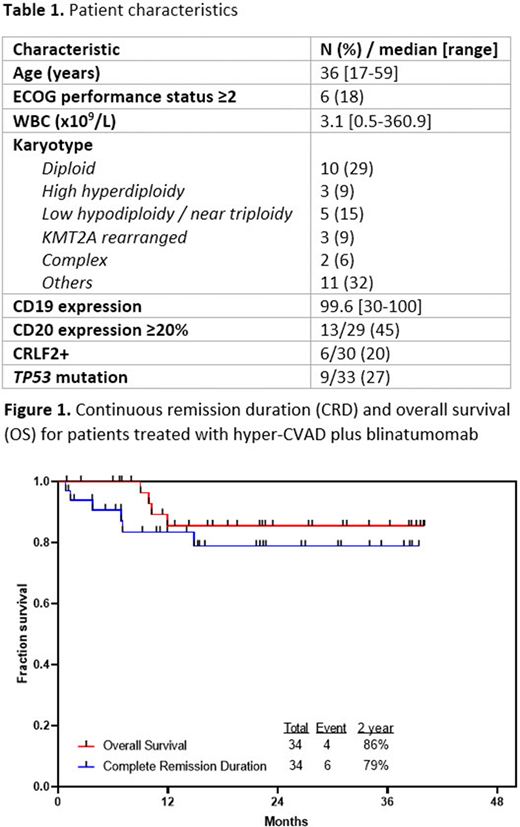
With a median follow-up of 22 months (range, 1-40 months), the 2-year continuous remission and OS rates were 79% and 86%, respectively (Figure 1). Overall, 5 pts (15%) relapsed, 12 (35%) underwent allogeneic SCT in first remission (including 1 additional pt who relapsed post-SCT), and 17 (50%) remain in continuous remission and are currently on treatment or have completed maintenance. All relapses occurred in pts with established poor-risk features, including 2 pts with KMT2A rearrangement, 2 pts with TP53 mutation (1 of whom was low hypodiploid), and 1 pt with baseline WBC count of 32 x 109/L. Two of these relapses occurred during hyper-CVAD cycles before the amendment allowing for earlier integration of blinatumomab for pts with high-risk disease features.
Treatment was overall well-tolerated. Four pts developed grade 2-3 cytokine release syndrome (grade 2, n=3; grade 3, n=1) which resolved with corticosteroids and interruption of blinatumomab. Overall, 14 (41%) pts had a neurological AE of any grade due to blinatumomab. Only one pt discontinued blinatumomab due to blinatumomab-related adverse event (grade 2 encephalopathy and dysphasia).
Conclusion: Sequential combination of hyper-CVAD and blinatumomab is highly effective as frontline treatment of Ph-negative B-cell ALL, with a CR rate of 100% and 97% of pts achieving MRD negativity. Survival data are promising with an estimated 2-year OS of 86%, which compares favorably to historical controls. This study continues to accrue pts.
Short:Astellas: Research Funding; Amgen: Honoraria; AstraZeneca: Consultancy; Takeda Oncology: Consultancy, Honoraria, Research Funding. Kantarjian:Jazz: Research Funding; Novartis: Honoraria, Research Funding; Pfizer: Honoraria, Research Funding; Daiichi-Sankyo: Honoraria, Research Funding; Oxford Biomedical: Honoraria; Aptitute Health: Honoraria; BioAscend: Honoraria; Delta Fly: Honoraria; Janssen: Honoraria; Actinium: Honoraria, Membership on an entity's Board of Directors or advisory committees; Immunogen: Research Funding; BMS: Research Funding; Adaptive biotechnologies: Honoraria; Abbvie: Honoraria, Research Funding; Sanofi: Research Funding; Amgen: Honoraria, Research Funding; Ascentage: Research Funding. Ravandi:Astellas: Consultancy, Honoraria, Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Macrogenics: Research Funding; AstraZeneca: Consultancy, Honoraria; Jazz Pharmaceuticals: Consultancy, Honoraria, Research Funding; Xencor: Consultancy, Honoraria, Research Funding; Orsenix: Consultancy, Honoraria, Research Funding; Abbvie: Consultancy, Honoraria, Research Funding; Celgene: Consultancy, Honoraria; BMS: Consultancy, Honoraria, Research Funding. Kadia:Abbvie: Honoraria, Research Funding; BMS: Honoraria, Research Funding; Cyclacel: Research Funding; Astellas: Research Funding; Cellenkos: Research Funding; Pfizer: Honoraria, Research Funding; Celgene: Research Funding; Pulmotec: Research Funding; Amgen: Research Funding; Genentech: Honoraria, Research Funding; Ascentage: Research Funding; Incyte: Research Funding; JAZZ: Honoraria, Research Funding; Novartis: Honoraria; Astra Zeneca: Research Funding. Thompson:Pharmacyclics: Research Funding; Adaptive Biotechnologies: Consultancy, Research Funding; Genentech: Consultancy; Janssen-Cilag: Honoraria; AbbVie: Research Funding. Alvarado:Tolero Pharmaceuticals: Research Funding; Daiichi-Sankyo: Research Funding; Astex Pharmaceuticals: Research Funding; Jazz Pharmaceuticals: Research Funding; Sun Pharma: Research Funding; BerGenBio ASA: Research Funding; MEI Pharma: Research Funding; FibroGen: Research Funding. Jain:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Precision Bioscienes: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Fate Therapeutics: Research Funding; AstraZeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; BMS: Research Funding; Pfizer: Research Funding; ADC Therapeutics: Research Funding; Incyte: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Cellectis: Research Funding; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; TG Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; BeiGene: Honoraria, Membership on an entity's Board of Directors or advisory committees; Aprea Therapeutics: Research Funding. Yilmaz:Pint Pharma: Honoraria; Pfizer: Research Funding; Daicho Sankyo: Research Funding. Konopleva:Calithera: Research Funding; Genentech: Consultancy, Research Funding; Amgen: Consultancy; Reata Pharmaceutical Inc.;: Patents & Royalties: patents and royalties with patent US 7,795,305 B2 on CDDO-compounds and combination therapies, licensed to Reata Pharmaceutical; Agios: Research Funding; AstraZeneca: Research Funding; Cellectis: Research Funding; Stemline Therapeutics: Consultancy, Research Funding; Forty-Seven: Consultancy, Research Funding; Rafael Pharmaceutical: Research Funding; Eli Lilly: Research Funding; AbbVie: Consultancy, Research Funding; Ascentage: Research Funding; F. Hoffmann La-Roche: Consultancy, Research Funding; Kisoji: Consultancy; Sanofi: Research Funding; Ablynx: Research Funding. Garcia-Manero:AbbVie: Honoraria, Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Astex Pharmaceuticals: Consultancy, Honoraria, Research Funding; Acceleron Pharmaceuticals: Consultancy, Honoraria; Amphivena Therapeutics: Research Funding; Jazz Pharmaceuticals: Consultancy; Merck: Research Funding; H3 Biomedicine: Research Funding; Helsinn Therapeutics: Consultancy, Honoraria, Research Funding; Novartis: Research Funding; Genentech: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Onconova: Research Funding. O'Brien:Amgen, Astellas, Celgene, GlaxoSmithKline, Janssen Oncology, Aptose Biosciences Inc. Vaniam Group, AbbVie, Alexion, Verastem, Eisai, Juno Therapeutics, Vida Ventures: Consultancy; Kite, Regeneron, Acerta: Research Funding; Gilead, Pharmacyclics, TG Therapeutics, Pfizer, Sunesis: Consultancy, Research Funding. Jabbour:Takeda: Other: Advisory role, Research Funding; AbbVie: Other: Advisory role, Research Funding; Pfizer: Other: Advisory role, Research Funding; Genentech: Other: Advisory role, Research Funding; BMS: Other: Advisory role, Research Funding; Adaptive Biotechnologies: Other: Advisory role, Research Funding; Amgen: Other: Advisory role, Research Funding.
Blinatumomab - frontline therapy for ALL
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal